Influenza Vaccine 2021 Ingredients
Influenza virus vaccine 2021-2022 quadrivalent - TasmaniaVictoriaPhuketWashington 05 ML Prefilled Syringe. Due to manufacturer price increases prices are subject to change without notification.

Fluarix Tetra Nps Medicinewise
Most will be thimerosal-free or thimerosal-reduced vaccine 87 and about 18 of flu vaccines will.

Influenza vaccine 2021 ingredients
. Overview 26 February 2021. 012021 MRC-5 cells including DNA and protein sucrose hydrolyzed gelatin sodium chloride sorbitol monosodium L-glutamate sodium phosphate dibasic recombinant human albumin sodium bicarbonate potassium phosphate monobasic potassium chloride potassium phosphate dibasic neomycin bovine calf serum other buffer and media ingredients. Polyethylene glycol-2000-NN-ditetradecylacetamide 12-Distearoyl-sn-glycero-3-. Influenza Vaccine Information Department Of Health.However due to high demand for influenza vaccination in some locations there may be some limited or temporarily unavailable supplies of specific types of needles and needlesyringe sets. The committee recommended that the quadrivalent formulation of egg-based influenza vaccines for the US. Composition Lot Release and Other Seasonal Information. It is recommended that quadrivalent vaccines for use in the 2021 - 2022 northern hemisphere influenza season contain the following.
Vaccine manufacturers have projected that they will supply the United States with as many as 188 million to 200 million doses of influenza vaccine for the 2021-2022 season. The COVID-19 vaccines are all inactive. Agustus 31 2021 Posting Komentar The key ingredient in all vaccines is one or more active ingredients see below. Phosphocholine and cholesterol potassium chloride monobasic potassium.
An AVictoria25702019 H1N1pdm09-like virus. See package insert for quantities Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed Hepatitis B Recombinant and Inactivated Poliovirus Vaccine Combined. Each year the Food and Drug Administrations Vaccines and Related Biological Products Advisory Committee VRBPAC makes the recommendation for the flu vaccine composition for US flu vaccines. Influenza vaccine 2021-22.
Influenza Virus Vaccine Composition and Lot Release information older than 2 years is available on FDA Archive. A BWashington022019 BVictoria lineage-like virus. Influenza vaccines United States 202021 influenza season Trade name Manufacturer Presentation Age indication HA IIVs and RIV4 or virus count LAIV4 for each vaccine virus per dose Route Mercury from thimerosal μg05mL. Influenza Vaccine for the 2020-2021 Season.
2020 INDICATIONS AND USAGE FLUAD QUADRIVALENT is an inactivated influenza vaccine indicated for active immunization against influenza disease caused by influenza virus subtypes A and types B contained in the vaccine. Researchers make this prediction. The influenza A virus H1N1 also known as. All flu vaccines for the 2021-2022 season will be quadrivalent four component.
It is recommended that quadrivalent vaccines for use in the 2021 - 2022 northern hemisphere influenza season contain the following. Standard dose egg based Afluria Quadrivalent Seqirus 025-mL PFS 6 through 35 mos. Cell- or recombinant-based Vaccines. For 2021-2022 recommendations were made for egg-based cell-based and recombinant flu vaccines.
Adequate supplies are expected to be available to support both the 20202021 influenza vaccination program and routine vaccination efforts. 1 Infection can be caused by both A and B strains of the influenza virus. HealthDayLicensed and age-appropriate vaccines for influenza are recommended for the 2021 to 2022 season for all persons aged 6 months. A single 05 mL dose of Influenza.
2021-2022 influenza season contain the following. MRNA lipids 4-hydroxybutylazanediylbis hexane-61-diylbis 2-hexyldecanoate 2. FLUARIX QUADRIVALENT 2021-2022 vaccine 05 mL Prefilled Syringe. The full list of ingredients for the Pfizer vaccine is.
VACCINE INGREDIENTS not in order of quantity. An ACambodiae08263602020 H3N2-like virus. A BPhuket30732013 BYamagata lineage-like virus. Click on the link to go to the Administration of influenza vaccines webpage.
These projections may change as the season progresses. How much influenza vaccine is. Influenza Vaccine for the 2021-2022 Season. FLUAD QUADRIVALENT Influenza Vaccine Adjuvanted Injectable Emulsion for Intramuscular Use 2021-2022 Formula Initial US.
As COVID-19 vaccination efforts continue it is more important than ever for pharmacists to be equipped with the knowledge and resources to bust the myths surrounding vaccinations particularly in preparation for the upcoming influenza season which is expected to occur from October 2021 to May 2022. The influenza viruses contained in the trivalent 20202021 flu vaccine are.

Fluarix Tetra Nps Medicinewise

Influenza Vaccine Information Department Of Health

2020 2021 Flu Shot Ingredients What Is In The Flu Shot And Why Fatherly

Fluarix Tetra Nps Medicinewise

Vaxigrip Tetra Nps Medicinewise
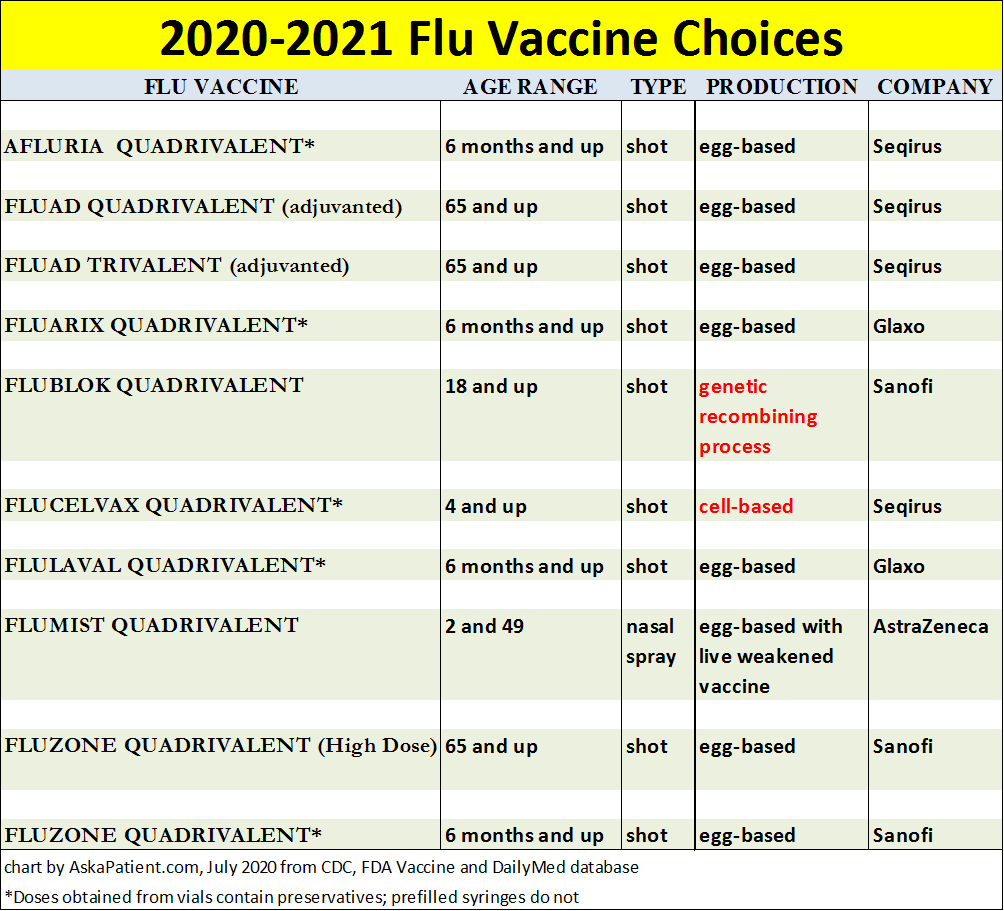
Flu Shot Options For 2020 2021 Amid Covid 19 Askapatient Com
Flu Shot Ingredients Includes Some Of These Or Do They I Speak Of Dreams
The influenza A virus H1N1 also known as. Composition Lot Release and Other Seasonal Information.

2020 2021 Flu Shot Ingredients What Is In The Flu Shot And Why Fatherly
FLUAD QUADRIVALENT Influenza Vaccine Adjuvanted Injectable Emulsion for Intramuscular Use 2021-2022 Formula Initial US.
Influenza vaccine 2021 ingredients
. Adequate supplies are expected to be available to support both the 20202021 influenza vaccination program and routine vaccination efforts. These projections may change as the season progresses. Overview 26 February 2021. An AVictoria25702019 H1N1pdm09-like virus.Each year the Food and Drug Administrations Vaccines and Related Biological Products Advisory Committee VRBPAC makes the recommendation for the flu vaccine composition for US flu vaccines. Researchers make this prediction. The influenza viruses contained in the trivalent 20202021 flu vaccine are. A single 05 mL dose of Influenza.
Influenza vaccines United States 202021 influenza season Trade name Manufacturer Presentation Age indication HA IIVs and RIV4 or virus count LAIV4 for each vaccine virus per dose Route Mercury from thimerosal μg05mL. However due to high demand for influenza vaccination in some locations there may be some limited or temporarily unavailable supplies of specific types of needles and needlesyringe sets. As COVID-19 vaccination efforts continue it is more important than ever for pharmacists to be equipped with the knowledge and resources to bust the myths surrounding vaccinations particularly in preparation for the upcoming influenza season which is expected to occur from October 2021 to May 2022. Influenza Vaccine for the 2020-2021 Season.
Phosphocholine and cholesterol potassium chloride monobasic potassium. All flu vaccines for the 2021-2022 season will be quadrivalent four component. 2020 INDICATIONS AND USAGE FLUAD QUADRIVALENT is an inactivated influenza vaccine indicated for active immunization against influenza disease caused by influenza virus subtypes A and types B contained in the vaccine. See package insert for quantities Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed Hepatitis B Recombinant and Inactivated Poliovirus Vaccine Combined.
HealthDayLicensed and age-appropriate vaccines for influenza are recommended for the 2021 to 2022 season for all persons aged 6 months. Cell- or recombinant-based Vaccines. A BPhuket30732013 BYamagata lineage-like virus. An ACambodiae08263602020 H3N2-like virus.
Influenza Vaccine for the 2021-2022 Season. FLUARIX QUADRIVALENT 2021-2022 vaccine 05 mL Prefilled Syringe. Polyethylene glycol-2000-NN-ditetradecylacetamide 12-Distearoyl-sn-glycero-3-. VACCINE INGREDIENTS not in order of quantity.
1 Infection can be caused by both A and B strains of the influenza virus. Vaccine manufacturers have projected that they will supply the United States with as many as 188 million to 200 million doses of influenza vaccine for the 2021-2022 season. For 2021-2022 recommendations were made for egg-based cell-based and recombinant flu vaccines. 012021 MRC-5 cells including DNA and protein sucrose hydrolyzed gelatin sodium chloride sorbitol monosodium L-glutamate sodium phosphate dibasic recombinant human albumin sodium bicarbonate potassium phosphate monobasic potassium chloride potassium phosphate dibasic neomycin bovine calf serum other buffer and media ingredients.
Influenza Virus Vaccine Composition and Lot Release information older than 2 years is available on FDA Archive. It is recommended that quadrivalent vaccines for use in the 2021 - 2022 northern hemisphere influenza season contain the following. Agustus 31 2021 Posting Komentar The key ingredient in all vaccines is one or more active ingredients see below. MRNA lipids 4-hydroxybutylazanediylbis hexane-61-diylbis 2-hexyldecanoate 2.
The COVID-19 vaccines are all inactive. The full list of ingredients for the Pfizer vaccine is. A BWashington022019 BVictoria lineage-like virus. The committee recommended that the quadrivalent formulation of egg-based influenza vaccines for the US.
Influenza vaccine 2021-22. Influenza Vaccine Information Department Of Health. Standard dose egg based Afluria Quadrivalent Seqirus 025-mL PFS 6 through 35 mos. Click on the link to go to the Administration of influenza vaccines webpage.
2021-2022 influenza season contain the following. It is recommended that quadrivalent vaccines for use in the 2021 - 2022 northern hemisphere influenza season contain the following. How much influenza vaccine is.

Fluarix Tetra Nps Medicinewise

Fluarix Tetra Nps Medicinewise

Vaxigrip Tetra Nps Medicinewise

Flu Shot Options For 2020 2021 Amid Covid 19 Askapatient Com
Flu Shot Ingredients Includes Some Of These Or Do They I Speak Of Dreams

Posting Komentar untuk "Influenza Vaccine 2021 Ingredients"